- • Basic tissues
- – Gingiva
- – Cementum
- – Periodontal ligament
- – Alveolar bone
- • Cell organelles
- • Cells of the periodontium
- – Keratinocytes
- – Fibroblasts
- – Cementoblasts
- – Bone cells
- – Epithelial cell rests of malassez
- – Stem cells
BASIC TISSUES
The periodontium comprises of two soft tissues and two hard tissues. The soft tissues are gingiva and periodontal ligament and the hard tissues are cementum and alveolar bone. The tooth supporting structures are the cementum, periodontal ligament and alveolar bone. Gingiva covers the alveolar bone and surrounds the tooth in a collar like manner. These structures function together as a group and thereby enable the tooth to carry out all its functions. The histological features of each tissue are designed to meet their specific functions.
A very brief overview of the gross anatomy, the histological features and their functions must be understood before the molecular processes that determine these features are discussed. A brief description of the pathological changes affecting the structures will follow, after which the molecular process that mediate these changes will be discussed.
Gingiva
Gingiva covers the alveolar process of the jaw and is anatomically divided into free gingiva, attached gingiva and interdental gingiva. Histologically, gingiva consists of epithelium and connective tissue, and the gingival epithelium is further subdivided into three types-oral gingival epithelium, sul-cular epithelium and junctional epithelium.
Free gingiva or marginal gingiva
Marginal gingiva extends from the crest of the gingiva to the free gingival groove which separates the marginal gingiva from the attached gingiva. The marginal gingiva is called ‘free’ as it is not attached to the underlying periosteum of alveolar bone. Coronally, it extends upto 1–2 mm coronal to the cementoenamel junction while apically it extends to the free gingival groove. The free gingival groove is not usually clinically visible and the apical extent of the marginal gingiva is generally determined by probing depth. The 2apical extent of the probe corresponds to the level at which gingiva is attached to tooth. In health, it is at the level of the cementoenamel junction and this attachment is mediated by the junctional epithelium.
Functionally, the marginal gingiva forms the soft tissue wall of the V-shaped gingival sulcus that is formed by the tooth on one side and the gingiva on the other. The gingival sulcus can be deflected away from tooth by a blunt instrument such as the periodontal probe. In health, the gingival sulcus has a probing depth of about 2–3 mm. The sulcus is filled with the inflammatory exudate called the gingival crevicular or sulcular fluid (GCF). The gingival crevicular fluid has in addition to other constituents, factors important for the defense mechanism of gingiva. The need for defense arises from the fact that the sulcus is the primary plaque forming domain and is the portal of entry for periodontopathogenic bacteria. The gingival fibers help brace the marginal gingiva and prevent it from getting overly displaced from the tooth. Marginal gingiva is thus equipped with cellular and extracellular matrix elements that grant both esthetic and functional characteristics.
Attached gingiva
The attached gingiva is firmly attached to the periosteum of the underlying alveolar bone. It extends from the free gingival groove to the mucogingival junction apically. The attached gingiva is thus firmly entrenched between two movable structures-the marginal gingiva coronally and the alveolar mucosa apically. The dense connective tissue fibers of the attached gingiva ensure its firm attachment to the bone and grant it the required “toughness”.
The dimensions of the attached gingiva vary from the anterior to the posterior teeth. The width of the attached gingiva refers to its apico-coronal dimension and varies from 3.5–4.5 mm in the anterior teeth to 1 mm in the premolar region. The width is often least in the premolar regions due to the presence of accessory frenae in those areas. In the palatal surface, the attached gingiva blends almost imperceptibly to the palatal mucosa. Lingually, the attached gingiva gives way to the lingual mucosa and is dimensionally much lesser than on the buccal surface.
The width of attached gingiva increases with age and in supraerupted teeth. This increase in dimension occurs as a result of an increase in the height of the alveolar process which in turn is the result of passive eruption. The width of the attached gingiva is reduced either due to gingival recession or periodontal pocket formation.
The mucogingival line remains stationary throughout life as it is a genetically determined landmark that separates not only the attached gingiva from the alveolar mucosa, but also the alveolar process from the basal bone. Any change in dimension is thus, a direct result of changes occurring in the gingiva.
The other dimension that may play a significant role in the maintenance of the periodontal health is the thickness of the gingiva. Gingival phenotype or biotype has been classified by Eger & Muller into thick and thin or Class I, IIA and IIB. Thick gingival phenotype seems to be more conducive to periodontal health. A thin phenotype predisposes to gingival recession and increased tendency to gingival inflammation.
The functional relevance of attached gingiva relates to its firm attachment to the underlying bone. It prevents deflection of marginal gingiva due to the effects of lip musculature and frena. By preventing this deflection, it also helps avoid food entrapment 3between tooth and gingival sulcus, thus aids plaque maintenance. The dense collagenous structure of the attached gingiva also aids in slowing down the inflammatory spread from the marginal gingiva.
Clinical features of gingiva
The clinical features of gingiva are a reflection of the underlying molecular process. The form and function of gingiva is determined by the molecular process contributing to it.
Color
Normal gingiva is coral pink in color. Factors that affect the color of the gingiva are the degree of keratinization, vascularity and pigmentation. Vascularity determines the basic color of gingiva which undergoes modification depending on the degree of keratinization and presence of pigments. Melanin pigments form the major portion of the pigmented material present in the gingiva and results in dark color. The molecular processes that are involved in keratinization and melanin pigmentation are related to the cytoskeletal elements of the cells.
Contour
Normal gingiva has an arcuate contour with a scalloped outline and a knife edge margin. The contour is dictated almost entirely by the alveolar bone which follows a positive architecture, i.e. interdental bone is at a coronal position when compared to the labial bone. The soft tissue pattern is invariably dictated by that of the hard tissue.
Consistency
In health, gingiva is firm and resilient. Firm consistency is a result of the cellular and fibrous content of the extracellular matrix. The abundant collagen fibers and the non-collagenous proteins combine to give gingiva the firm consistency that enables it to resist masticatory stresses.
Surface texture
The gingiva has a stippled appearance that has been described as that resembling an orange peel. The stippling occurs as a result of the epithelial retepegs in growth into the connective tissue. The papillary layer of connective tissue extends into the epithelium and this feature results in a stippled rather than a smooth surface. It is thought to be functional adaptation to increase resistance to mechanical stresses.
Size and shape
This corresponds to the shape of the teeth and subsequently alveolar bone. The bulk of the gingiva is composed of connective tissue comprising of cellular elements and extracellular matrix.
Position
Gingival margin is located coronal to the cementoenamel junction and rests on the enamel. The gingiva is attached through the junctional epithelium to the CEJ of the tooth.
The clinical features that have been described above are all a result of molecular processes that occur within the cell. These processes and the changes they undergo during pathology are described in subsequent chapters.
Histology
Gingival epithelium is of three types-the oral epithelium, sulcular epithelium and junctional epithelium. Each of these epithelia is present in different areas of the gingiva and 4is expected to perform specific functions. The oral gingival epithelium is keratinized and has cells that are in close contact with each other through cell junctions. The primary function of the oral gingival epithelium is to provide an impermeable mechanical barrier, impervious to both fluids and bacteria.
Sulcular and junctional epithelium are both non-keratinized. Sulcular epithelium lines the gingival sulcus. Junctional epithelium has special features to allow for adhesion to tooth. The junctional epithelium along with the gingival fibers forms the dentogingival unit which provides attachment to the tooth. In addition, junctional epithelium is also expected to exhibit defense capability against the invading microorg-anisms. The non-keratinized epithelial cells which are not in close contact with each other allow diffusion of the sulcular fluid from the connective tissue to fill the sulcus. The crevicular fluid with its flushing effect and anti bacterial properties helps keep the pathogenic processes in check.
Plaque related gingival inflammation can be broadly of two types-edematous or fibrotic. Edematous inflammation results in bluish red color, soft consistency and a smooth surface that bleeds on probing. Fibrotic inflammation causes gingiva to remain predominantly pink in color with firm consistency, retaining its stippling and perhaps some change in contour due to enlargement.
Gingivitis is a reversible condition and the pathology may be self-limiting as long as only the gingiva is involved. The factors that trigger the change from gingivitis to periodontitis have not yet been fully elucidated but are related to the host mediated response. Periodontitis is today, known to be a result of an exaggerated response to specific bacteria present in biofilm such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tanerella forsythus, etc. The distinctive changes in the periodontium occur as a result of host mediated damage. The end result of these changes is apical migration of the junctional epithelium leading to pocket formation. The molecular basis behind the process of apical migration is described in later chapters.
Cementum
Cementum is the mesenchymal tissue that covers the root surface. It provides attachment for the periodontal ligament to the root surface and is hence an integral part of the attachment apparatus. Although cementum resembles bone structurally, the avascular nature of this tissue and its subsequent low turn over makes it distinct from bone. Cementum is the least understood of all the four tissues of the periodontium.
Cementum has been classified into acellular and cellular cementum. Acellular cementum is present in the coronal 2/3rd of the root and is characterized by the lack of cells. Cellular cementum is present in the apical 1/3rd of the root surface and is characterized by the presence of the cementum forming cells called cementoblasts.
Cementum is also classified based on the fibers present in its matrix into intrinsic and extrinsic cementum. Extrinsic cementum consists of fibers that arise from the periodontal ligament and are inserted perpendicularly to the root surface. These fibers are mineralized and are called Sharpey's fibers. Sharpey's fibers are arranged perpendicular to the root surface and in regular bundles corresponding to the principal fibers of the periodontal ligament. Intrinsic fibers are secreted by the 5cementoblasts and are present within the matrix. These fibers are randomly arranged and are not present in bundles like the extrinsic fibers.
Cementum is thus classified as–
Acellular afibrillar cementum
This cementum is present just apical to the cementoenamel junction. It is devoid of both cellular and fibrillar elements. Instead, there is a cementoid matrix that is poorly differentiated and unorganized. This cementum forms a weak link which may have important clinical implications in that iatrogenic or inflammatory damage to this structure may result in rapid spread of inflammation to the pulp.
Acellular extrinsic fiber cementum
This form of cementum is present in the coronal portion of the root and is the most mineralized. This cementum provides the mechanical rigidity that is important for the attachment apparatus. Loss of this cementum as a result of periodontal disease results in loss of the most important attachment structure of the tooth.
Cellular mixed stratified cementum
This is a combination of both extrinsic and intrinsic fiber cementum with regularly arranged extrinsic fibers interspersed with the random intrinsic fibers.
Cellular intrinsic fiber cementum
Intrinsic fiber cementum is present in only the apical areas of the tooth. It is this cementum that undergoes continuous deposition throughout life. This deposition is important to compensate for the physiological attrition that necessitates continuous passive eruption.
Intermediate cementum
The presence of this type of cementum is somewhat controversial. Some investigators have claimed that this cementum is infact an experimental artefact comprising of a proteoglycan-glycoprotein layer. This cementum is formed as a result of failure of the hertwigs's epithelial root sheath to retract as a result of which the cementoblasts are unable to gain entry to this area.
Cementum was for long considered to be an inert mineralized tissue due of its avascular nature and lack of turn over. It is now recognized that, cementum is associated with several biological proteins that are involved in the attachment as well as homeostatic mechanisms.
Cementum attachment protein
Cementum attachment protein (CAP) is present on the outer surface of the cementum and mediates two important functions. CAP is known to play a role in chemotaxis and differentiation of cementoblasts prior to laying down of the cementoid matrix. In addition, CAP mediates the attachment of the periodontal ligament fibers to the root surface.
Cementum growth factor
It bears a structural homology to insulin like growth factor. It is a tyrosine phosphatase that is involved in the cell signaling processes that are thought to be involved in the differentiation of cementoblasts and hence play a role in organization and formation of cementum.
The biological characteristics of the acellular and cellular cementum are yet to be clearly delineated and offers considerable scope for further research. Similarly the origin and factors that affect the differentiation of 6cementoblasts are yet to be fully elucidated. Currently available information about these factors has been described in later chapters.
Periodontal ligament
Periodontal ligament is the mesenchymal tissue that is present between the cementum and alveolar bone. Periodontal ligament attaches the tooth to alveolar bone and absorbs the mechanical load that is applied during mastication. It consists of collagen bundles that are arranged in specific groups termed as the principal fibers of the periodontal ligament. The principal fibers are divided into groups as follows.
Transseptal fibers
These fibers start apical to the cementoenamel junction of one tooth, traverse the alveolar bone and are inserted into the cementum of the adjacent tooth. Transsseptal fibers are important for maintenance of spatial arrangement of teeth. They reorganize rapidly and once they form back, try to get teeth back to their original position.
Alveolar crest group
This group of fibers run from the cementum to the crest of the alveolar bone. These fibers resist any forces that tend to lift the teeth away from the socket, i.e. extrusive forces.
Oblique fibers
They are the most numerous of the principal fibers. They arise from the root and run in an oblique direction to the alveolar bone. These fibers bear the brunt of the masticatory forces subjected to the tooth.
Horizontal group
These fibers run from the root to the alveolar bone in a perpendicular direction to the long axis of the tooth. These fibers resist torsional forces and prevent any laterotrusive movement.
Apical group
Apical fibers run from the root apex to the alveolar bone. They are the most resistant among the principle fibers. These fibers are meant to resist intrusive forces.
Inter-radicular fibers
These fibers are present in multirooted teeth between two or three roots as the case may be. These fibers reinforce anchorage to bone.
The principal fibers are made of type I collagen. In addition to type I, the periodontal fibers have other collagens like type III, XVIII etc. In addition to collagen, periodontal ligament also has oxytalan fibers which belong to the elastin network (elastin, elaunin and oxytalan). Periodontal ligament also has a characteristic distribution of glycoproteins and proteoglycans present in its extracellular matrix.
Cellular elements present in the periodontal ligament are fibroblasts, osteoblasts, undifferentiated mesenchymal cells and the ECRM. The role of each of these cells, their unique features and the molecular process each of them undergo are described later in this chapter.
Functions
The functions of the periodontal ligament are physical, formative, sensory and nutritive. 7This book will deal with only the molecular aspects of all these functions. The mechanosensory signals are transmitted to specific receptors. The pathway of communication required for the receptors to function is described in the chapter “cell communication”. Similarly, the growth factors and their function in response to forces are also described in this chapter. A remarkable feature of the periodontal ligament is the maintenance of the periodontal ligament. Maintenance of this space is made possible through a number of factors like ECRM, nitric oxide, osteocalcin and other genetic factors, all of which have been described in later sections.
Alveolar bone
Alveolar bone is the “raison d'tere” for the existence of tooth in a way. Alveolar bone has been divided into alveolar bone proper and supporting alveolar bone. The alveolar bone proper refers to that part of the alveolar bone that bears the root of the teeth, while the supporting alveolar bone is the basal bone of the maxilla and mandible. Alveolar bone proper has also been divided into bundle bone and lamellated bone. Bundle bone is called so because of the insertion of numerous collagen bundles of the principle fibers of the periodontal ligament being inserted into it. Lamellated bone is that part of alveolar bone adjacent to bundle bone. Histologically, the appearance of osteoblasts arranged in concentric circles called lamella, led to the usage of this term. Radiographically, a thick radioopaque line is present around the root of the teeth. This is referred to as the lamina dura and is the radiographic reperesentation of alveolar bone proper.
The alveolar process is in most parts dictated by teeth. The shape of the root, alignment of teeth and their position in the arch determine the shape and size of alveolar bone. The bone cells are osteoblasts, osteoclasts and osteocytes, and together they regulate bone formation. These cells and their regulating functions are described later in this chapter. Bone loss is perhaps one of the most definitive features of periodontitis.
The characteristic features of each of these tissues have been described briefly. The intracellular process that goes towards formation and maintenance of these tissues in health, the changes they undergo during pathology and their potential use as markers of periodontal disease forms the rest of the book.
CELL ORGANELLES
Each tissue of the periodontium thus has its own distinct functional and biochemical characteristics that are a result of their matrix components. The synthesis and turn over of these matrix components are controlled by intracellular events in organelles that are specifically designed for this purpose.
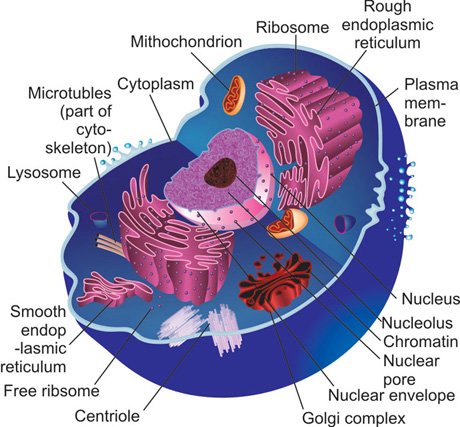
The structural organization of these intracellular organelles is basically similar in most eukaryotic cells (Fig. 1.1).
All cells are enclosed by a plasma membrane within which is the cytoplasm or the cytosol of the cell. Each cell has several structures called organelles that are required for synthesis, transport of substances (esp. proteins) and to perform important functions that are required for basic cell survival. The important organelles of the cell are-cytosol, nucleus, Golgi complex, endoplasmic reticulum, ribosomes and lysosomes. These organelles have been briefly discussed so as to offer an understanding of the common processes that are involved in production of structural molecules that are relevant to the periodontium.
Plasma membrane
The plasma membrane forms the outer boundary of the cell, thereby separating the cytosol from the external environment. As all cells are bathed in an aqueous environment, it is important to prevent ingress of water from the external environment into the cytosol. At the same time, the intracellular components (fluid) must be prevented form leaking out of the cell. The plasma membrane provides a barrier that prevents both the above mentioned events. The plasma membrane is however, not an inert rigid barrier.
The ability of cell to expand and adapt to their external stimuli is dependent on transfer of ions and solutes in and out of the cell. A rigid impermeable barrier would not allow exchange of material between the cell and the external environment. For this purpose, the plasma membrane needs to be semipermeable that would retain essential intracellular substances at the same time allow transfer of solutes that are required to maintain homeostasis.
Structure
Structurally, the plasma membrane consists of a lipid bilayer and a protein coat which help carry out its functions. The lipid bilayer provides the impermeability, while the proteins help in transfer of ions and other molecules in and out of the cell.
Unlike other membranes within the cell, plasma membrane needs to maintain its rigidity to prevent any distortion due to external pressure. This rigidity is conferred by the cytoskeleton.
All biological membranes that surround the cell and other intracellular organelles have a common general structure – an outer membrane and an inner membrane (Fig. 1.2). The lipid bilayer confers impermeability to the plasma membrane. This lipid bilayer has a hydrophilic end that is exposed to the external environment and a hydrophobic end that faces the interior of the cell. Apart from the lipids, the chief constituents of the plasma membrane are proteins.
These proteins link the lipid bilayer to the cytoskeleton as well as to adjacent cells. Further, these proteins are involved in all the functions of the cell membrane such as ion transport, ATP synthesis and receptor functions.
Membrane lipids
The lipids found in the plasma membrane are of three types–
- Phospholipids,
- Cholesterol, and
- glycolipids.
Phospholipids
The phospholipids are the most abundant among all the lipids present in the plasma membrane. They have a hydrophilic protein head and two hydrophobic tails of variable length. The tail portion of these molecules contains largely nonpolar fatty acids. While the hydrophilic polar end readily dissolves in water because of its charged surface; the non-polar tails do not interact with water molecules because of lack of any charge. The important phospholipids present in the cell membrane are phosphotidyl choline, phosphotidyl ethanolamine, phosphotidyl serine and sphingomyelin. Other phospholipids such as inositol phospholipids are present in much lesser proportions. Although present in small quantities, these phospholipids are functionally important as they play an important role in the cell signaling process.
Cholesterol
The cholesterol molecules present in the plasma membrane are aligned along the phospholipids with their hydroxyl groups joined to the polar ends of the phospholipids. The presence of cholesterol enhances the water impermeability of the plasma membrane.
Glycolipids
Glycolipids are sugar containing lipid molecules that exhibit extreme asymmetry in their distribution. They are found only in the non-cytosolic monolayer of the membrane, i.e., the outer membrane. In epithelial cells, these glycolipids are found only in the apical surface. Other important glycolipids are those that bind the surfaces of adjacent cells together.
The presence of the glyco lipids on the apical surface of the epithelial cells grants these cells their polarity. Cell polarity is important for the motility of the keratinocytes as we shall see in following chapters.
The lipid bilayer is not similar to each other and there is considerable asymmetry in their protein binding capability. This asymmetry has biological effects that are functionally relevant to the cell. There are specific proteins that bind to phospholipids that are asymmetrically distributed on each of the lipid layer, e.g., protein kinase C (PKC) binds to the hydrophobic tail of the lipid monolayer, specifically to phosphotidyl serine. The negative charge produced by the phosphotidyl serine is required for this binding to take place. On the other hand, phosphotidyl inositol is not present in the hydrophobic portion of the lipid bilayer. Following signal activation, this molecule transforms its binding to the hydrophilic portion of the membrane.
Membrane proteins
Proteins are an integral part of membrane complex and regulate their activities in various ways. Like their lipid counterparts, 10the proteins too have a hydrophobic and a hydrophilic end. The hydrophobic ends are embedded within the lipid bilayer, while the hydrophilic ends are exposed to the aqueous environment both inside and outside the cell. Within the cytosol, the hydrophilic ends are kept sequestered away from water so that they are not dissolved in it in normal physiological states. Other proteins do not traverse the membrane bilayer entirely and they may be associated only with the inner lipid layer through covalent bonds or other fatty acid chain attachments. Membrane proteins can be broadly classified into
- Integral membrane proteins, and
- Peripheral membrane proteins.
Types of membrane proteins
Integral membrane proteins
These proteins traverse almost the entire length of the lipid bilayer and cannot be separated from the membrane without causing damage to the lipid structure. This is because of the strong union these transmembrane proteins form either with other proteins or lipids present in the membrane. Transmembrane proteins, e.g. the cell surface receptors, have polypeptide chains that run across the lipid bilayer and these chains protrude on either side of the membrane. The polypeptide chain present on either side of the lipid bilayer can function almost independently of each other, e.g. the cell surface proteins have the ligand binding site forming the exterior of the cell and serve to bind to the signals. The same protein has a cytosolic portion that extends into the cytoplasm which serves to activate the cell signaling pathways.
Transmembrane proteins are usually present in two forms-the α helix and the β sheet. The α helix may be a single pass structure, i.e. they may cross the plasma membrane just once, e.g. receptor tyrosine kinases like the epithelial growth factor receptor, fibroblast growth factor receptor etc. They may otherwise be a multiple pass transmembrane structure like the G protein coupled receptors that run back and forth across the membrane seven times.
The β sheet proteins form transport channels that are mostly involved in transport of ions and other molecules across the cell. Transport proteins are classified into two main categories-
- The channel proteins, and
- The carrier proteins.
Channel proteins
These proteins are basically involved in passive transport of ions across the membrane along the concentration gradient. These channel proteins are formed as small aqueous pores within the lipid bilayer that allow ions to diffuse through them– a process that is described as facilitated diffusion.
Carrier proteins
These proteins are involved in active transport. They are able to carry ions across the concentration gradient, i.e., from a lower concentration to a higher concentration. This process of active transport is a more complicated system when compared to passive transport and requires energy. The active transport proteins are sub-classified into
- Coupled carriers
- ATP driven pumps.
Coupled carriers
These carrier proteins carry an ion from one side of the cell to the other. Uniporters are those proteins that are involved in transport 11of one ion without involving other ions in the process. Symporters carry one ion along with another ion, e.g. Na+ and Cl− ions. Antiporters are those that are involved in transport of one ion from inside to outside the cell while at the same time carrying another protein from outside to inside the cell, e.g. the Na+ and K+ ions. These carrier proteins along with the ATP driven pumps are important for maintaining the osmolarity of the cell which is essential for homeostasis.
Mitochondria
All eukaryotic cells require energy for production of proteins, transport of proteins from nucleus to cytosol and transport of macromolecules into and outside the cell. Mitochondria is the power house of the cell that provides the ATP and GTP that helps meet the energy requirement of the cell. Of the two, most energy processes within the cell utilize ATP. These energy molecules are produced largely from carbohydrates and lipids obtained form dietary sources. Proteins present in the diet are broken down into amino acids that are usually utilized for protein formation for other purposes.
The most important source of ATP is the dietary carbohydrates. These carbohydrates, are broken down to smaller subunits before they can be utilized by the cell through various metabolic processes.
Carbohydrate metabolism differs according to the presence and absence of oxygen. In the absence of oxygen, they undergo a pathway called the anaerobic glycolysis, whereby glucose is broken down into acids such as lactic acid. This process is generally not a rich a source of ATP. Oxidative phosphorylation, on the other hand, results in metabolism of glucose with formation of pyruvate as an end product of this metabolism. Several ATP molecules are produced during these reactions and these can be utilized for energy dependent processes within the cell. Even this process is however, not entirely energy efficient, as the pyruvate that is formed is not normally utilized for other purposes. Utilization of pyruvate and production of further molecules is possible only through the presence of specialized structures in the cell called the mitochondria.
Structure
Mitochondria are among the larger organelles present in the cell and is visible even through the light microscope. Structurally it has been described as resembling bacteria with extensions in its outer wall. Under higher magnification, it was realized that these extensions seen on mitochondria were not rigid structures. Mitochondria were a plastic structure which demonstrated the ability to change their surface characteristics with great ease and this was the reason for extensions observed under the light microscope. Mitochondria are thus able to change shape, fuse with each other and to other intracellular organelles such as the microtubule assembly. The number of mitochondria depends on the state of the cell, i.e. a cell that is more actively involved in protein synthesis tends to demonstrate a greater number of mito-chondria. For example, a liver cell which is actively involved in synthesis, demonstrates about 100–200 mitochondria.
Ultrastructurally, the mitochondria have two membranes called the inner and outer mitochondrial membrane with an intervening space labelled as the membrane space. The inner mitochondrial membrane encloses the mitochondrial matrix. The outer membrane is, by and large, a smooth structure but it is intersperced by large aqueous channels at 12several points. These large channels that are formed are called porins (Fig. 1.3). Porins are freely permeable to transport of most proteins that are of a relatively low molecular weight, i.e. lesser than 5000 Da. These proteins freely cross the outer membrane but are trapped within the membrane space.
The inner membrane is not a smooth structure like that of the outer membrane. They are thrown into several infoldings called the cristae. The inner membrane and the mitochondrial matrix are the structurally important regions of the mitochondria. The structural relevance of the matrix results from the fact that it is the site of the ‘citric acid cycle’ or ‘Kreb cycle’. Kreb cycle is, perhaps, the most important carbohydrate utilization process of the cell resulting in the formation of the carbon dioxide and water. During this process, several molecules of ATP are generated and this is then utilized for the energy requirement of the cell.
The other important feature of mitochondria is the fact that it is an important source of extra nuclear genetic material. While the overwhelming majority of the genetic material is stored in the nucleus, eukaryotic cells can exhibit DNA in other intracellular organelles. Among these organelles, mitochondria store a large amount of DNA, RNA polymerases and other nuclear proteins. The reason for such a large presence of genetic material in the mitochondria is currently unknown. However, it has been well characterized that inheritance of mitochondrial DNA does not follow mendelian laws like the nuclear DNA. Because of its nature of inheritance, the mitochondrial DNA lead to progeny with same haploid characteristics as that of the parent. This feature has been used to identify early genetic damage that occurs as a result of exogenous damage through radiation or chemicals. Oxidative damage, that is cellular damage that results from excessive production of reactive oxygen species, can be first recognized from mitochondrial DNA. Mitochondria are thus, not only the energy power house of the cell, but also an important source of extra nuclear genetic material.
Nucleus
It is the largest organelle in the cell and is the major store house of genetic material of the cell. The genetic basis of protein formation and the various genetic mechanisms that are important for the regulation of periodontium in health and disease are discussed in detail in later chapters. This important characteristic of the nucleus is not discussed here in this chapter.
The functions of the nucleus and the cytosol are, interdependent on each other. Optimal function of one organelle is dependent on proteins/macromolecules present in the other. In order to maintain homeostasis, there is a need for constant communication between the two 13compartments. Therefore, the nucleus and the cytosol are always organized in a sort of two way traffic system. The importance of this two-way communication can be illustrated from the following examples. Important proteins like histones and RNA polymerases that are required for nucleic acid regulation within the nucleus are normally present in the cytosol. Similarly, protein synthesis begins in the nucleus and is completed in the cytoplasm. This bidirectional trafficking is made possible through a network of open channels. Transport, both in and out of the nucleus occurs through an active transport process.
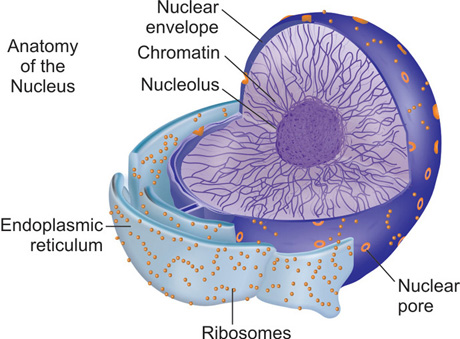
The nucleus is covered by the nuclear envelop that separates the nucleus and cytosolic components into two. The nuclear envelope has an outer nuclear membrane and an inner nuclear membrane which are structurally dissimilar to each other (Fig. 1.4). The outer nuclear membrane is continuous with the membrane of the endoplasmic reticulum at various points. The outer nuclear membrane has numerous ribosomes embedded on its surface. Inner nuclear membrane, on the other hand, has specific proteins that can bind to genetic material and transport them to the cytosol. The space between the inner and outer nuclear membrane, called the perinuclear space is continuous with the lumen of the endoplasmic reticulum.
The nuclear membrane is lined by large open structures called the ‘nuclear pore complexes’. These nuclear pore complexes are built by proteins called nucleoporins that are arranged in a symmetrical octagonal manner. Usually, the greater the transcriptional activity of the cell, the greater the number of pores in the nuclear envelop. Each nuclear pore is composed of one or more aqueous channels. These channels allow smaller molecules (less than 5000 Da) to pass through freely. This passive diffusion allows molecules such as Histones to pass into the nucleus. Histones, as we shall see in detail later, are required to pack DNA to form chromatin. This passive diffusion allows a large number of histones to be transported into the nucleus in a short period of time. In a cell that is undergoing active synthesis, about 100 histones must be transported into the nucleus every minute. The larger molecules, however, cannot use this transport mechanism as the small pores do not allow them to pass freely from one side to the other, e.g. the RNA polymerases that have to enter the nucleus and the large ribosome bound proteins are far too large to passively diffuse through these pores. As these macromolecules are important for the normal functioning of the genetic transfer and protein synthesis, this requires an alternate transport mechanism. This is achieved through an active transport process.
Active transport
Active transport process involves specific proteins that bind to and carry macromolecules in and out of the nucleus. 14This is made possible through the presence of carrier proteins that traverse the lipid bilayer of the nuclear membrane. The large aqueous channels in the nuclear pores allow the proteins to pass through without unfolding ie., they are transported in the same state in which they were synthesized. This is in contrast to other organelles where proteins have to be unfolded before they can be transported into or out of their barrier membranes. Membranes that are transported across the nucleus are recognized by what has been termed the nuclear localization signals. These signals, normally consists of short sequences of amino acids rich in lysine and arginine. These signals are in turn recognized by receptors present in the nuclear envelope called the nuclear import receptors, one of which binds to the signal and the other to nuleoporins. These nucleoporins also exhibit specific sites that can be utilized for binding to the import protein. The commonly used binding sites are called ‘FG” repeat areas, rich in phenylalanine and glycine which then recognize and bind to the import proteins. In some cases, the import proteins do not bind directly to the signal. Instead, signal binding is mediated by a set of adaptor proteins which serve as a bridge between the import proteins and the signal.
Similarly transport of proteins outside the nucleus occurs though nuclear export proteins. These proteins are encoded by a set of genes that exhibit structural homology to that of the import proteins. Functionally, too, they are very similar to import proteins and the only difference they exhibit is in the direction of movement, i.e., they move molecules from inside to outside the nucleus. This bidirectional active transport is an energy dependent process that requires activation of GTP/GDP. This energy is produced by a molecule called Ras. Ras is a monomeric GTPase, i.e., it can split GTP to form GDP and Pi and in the process releases energy. Ras is present in both cytosol and nucleus and is essential for active transport across the nucleus. Ras is present as bound to GTP or GDP and like other signal molecules, alternates between conversion from an active to inactive state. This conversion is brought about by GAPs that convert Ras-GDP to Ras-GTP. GAP's are present in significantly higher concentration in the cytosol. In contrast, the GEF's convert the GTP-Ras to GDP-Ras and is present more in the nucleus. They promote the import and export in the appropriate direction.
Golgi complex
The Golgi complex is one of the largest and most regularly outlined structure of the cell. The Golgi complex was identified even with light microscope. The Golgi complex is particularly prominent in cells that are undergoing active synthesis. The endoplasmic reticulum and Golgi complex are important for transport of proteins from nucleus to other parts of the cell. The Golgi complex is located close to the nucleus near the centrosome of the cell.
Structure
The Golgi complex consists of a number of flattened membrane bound structures called cisternae. Normally, eukaryotic cells contain about six or seven cisternae that are bound together through tubular connections such that they form a single complex. The cisternae and the tubules together form the structural units of Golgi (Fig. 1.5).
For practical purposes, the Golgi complex may be divided into two cis face and a trans face both of which are divided into two distinct compartments.15
Each compartment has its assembly of cisternae and tubular structures. The cis face represents the areas through which proteins and lipids enter the Golgi, while the trans face is the area through which they exit.
Functional significance
Several proteins that are required for important biochemical reactions within and outside the cell are glycoprotiens and proteoglycans. Both classes of proteins require addition of carbohydrates to the proteins that are secreted by the nucleus-ER/ribosome complex. This addition of carbohydrates to the protein skeleton is accomplished in the Golgi complex.
Glycoprotein processing
The Golgi complex is involved in the addition of oligosaccharide chains to the glycoproteins. Oligosaccharide processing occurs in an orderly manner within the cisternal stacks starting from the cis face and ending in the trans face. This processing involves both removal and addition of sugars when complex oligosaccharides are added to the proteins. Otherwise, this process involves merely removal of sugars such as that seen in high mannose oligosaccharides.
Proteoglycan processing
Proteoglycans are also processed in Golgi complex as the glycosylation of the core proteins occur in the Golgi complex. In addition to this, the glycosaminoglycan side chains of the proteoglycans are sulfated in the Golgi complex. Sulfation confers negative charge to the proteoglycan and thus helps in hydration and maintenance of the extracellular matrix. Glycosylation of the glycoproteins and proteoglycans is an important step in protein processing. Generally, glycosylation prevents digestion of proteins by the proteases present within the cell. In addition, it may serve specific functions such as–
- Adhesion in E-cadherin
- Cell signaling
Process of glycosylation
Golgi complex is divided into cis, medial and trans compartments wherein there is an orderly glycosylation of the proteins. Modification of proteins occurs in each compartment in separate phases. This process has been proposed to occur through two mechanisms-vesicular transport and cisternal maturation. Vesicular transport mechanism 16occurs as a result of protein being transported along the Golgi through its tubular network. In this mechanism, it is assumed that the cisternae are immobile and remains in their space. According to the cisternal maturation theory, the cisternae are thought to be dynamic and move from one end of the complex to the other. As a result of this cisternal transport, the glycosylated proteins enter from the cis face and exit from the trans face after completion of their processing.
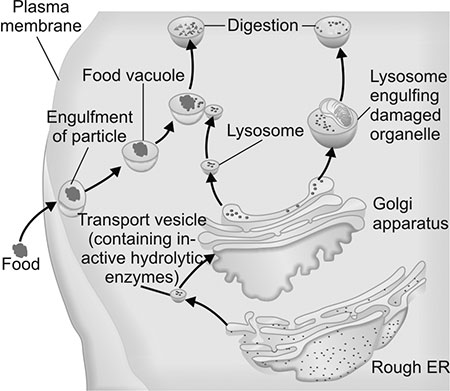
Lysosomes
After protein processing is completed in the Golgi, it is transported by the lysosomes to the exterior of the cell. Lysosomes are vesicles that contain lysosomal enzymes that are used to digest macromolecules (Fig. 1.6). Among the various enzymes present are nucleases, lipases, phospholipases etc. that are used to digest extracellular components. All these enzymes are acid hydrolases that function at a pH of 5 or less. If these hydrolytic enzymes were to gain entry into the cytosol, they would be capable of causing considerable damage to the intracellular structures. To prevent this form happening, the lysosomes are bound by a firm, non-porous membrane that prevents leakage of these enzymes into the cytosol. Additionally, the acid hydrolases function best in the acidic environment of the lysosomes and not in the alkaline environment of the cytosol. The glycosylated proteins are prevented from digestion by the lysosomal enzymes due to the presence of sugars bound to the protein.
Functional significance
Lysosomes are involved in endocytosis and exocytosis, which is the transport of macromolecules into and out of the cytosol respectively. In addition to this, the lysosomes are also involved in autophagy, i.e. digestion of intracellular macromolecules and sometimes organelles that have either outlived their usefulness or are malformed. Lysosomes thus provide the scavenger apparatus that is required to cleanse the cell from any undesirable material.
Endoplasmic reticulum
Endoplasmic reticulum (ER) is an important component of the intracellular organelle assembly. Its importance stems from the fact that the endoplasmic reticulum is an essential requisite for protein synthesis and transport process. For efficient functioning of this process, the ER membrane comprises about 50 percent of the total volume of the cell.
Structure
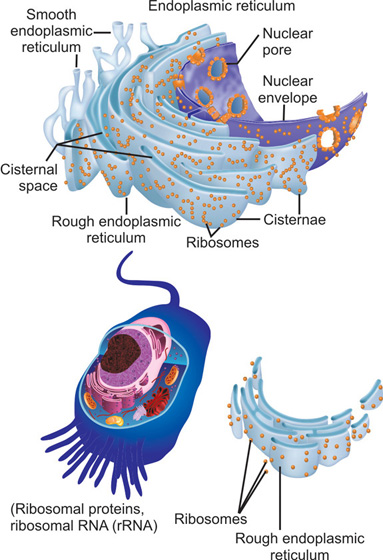
Endoplasmic reticulum consists of a labyrinthine network of tubules and sacs which are interconnected with each other (Fig. 1.7). As a result, it is thought to have a highly convoluted lumen.17
This lumen is called the cisternal space or the endoplasmic reticulum lumen. The cisternal space is separated from the cytosol by the endoplasmic reticulum membrane which acts as a selective barrier preventing the ingress and egress of molecules.
Functional relevance
Endoplasmic reticulum plays an essential role in synthesis of proteins and lipids. Most transmembrane proteins are synthesized in the endoplasmic reticulum. Similarly, the lipids that are an integral part of the membranes of other organelles such as mitochondria are also synthesized in the endoplasmic reticulum. The proteins and lipids are synthesized in various regions of the endoplasmic reticulum. Based on their structure they have been divided into two regions-rough endoplasmic reticulum (RER) and smooth endoplasmic reticulum (SER). Rough endoplasmic reticulum is so designated due to the presence of ribosomes attached to the endoplasmic reticulum. The ribosomes or the rough endoplasmic reticulum complex is entirely involved in protein synthesis. Areas of the endoplasmic reticulum that do not have ribosomes attached to its membrane are called the smooth endoplasmic reticulum and these are involved in lipid synthesis.
Protein synthesis begins in the nucleus and they are then secreted into the cytosol through the nuclear pores. The endoplasmic reticulum selectively captures these secreted proteins from the cytosol and internalizes them for further processing. These proteins are of two types, the transmembrane proteins and the water soluble proteins. The transmembrane proteins remain attached to the membrane of the endoplasmic reticulum, whereas the water soluble proteins cross the membrane barrier and enter the lumen. Functionally, these proteins differ in that the transmembrane proteins reside either in the endoplasmic reticulum itself or become an integral part of the membrane of other organelles, while the water soluble proteins remain in the lumen of other organelles or are secreted outside the cell.
Normally most proteins are transported into organelles after the process of translation is completed. Otherwise, the enzymes of the cytosol are capable of proteolysis of the polypeptide chains. In order to avoid this, 18proteins are folded and processed by molecules called chaperons that preserve the integrity of the proteins. Transport of proteins into most organelles is then a post-translational process. In contrast proteins are transported into ribosomes as a part of the translational process.
Ribosomes are classified into membrane-bound and free, both of which are structurally and functionally similar. Assembly of proteins, however, occurs in the ribosomes that are attached to the endoplasmic reticulum. Transport of proteins into the endoplasmic reticulum occurs when they are still in the stage of polypeptide chains and is thus a co-translational process. A single mRNA chain is bound by several ribosomes and the structure thus formed is called polyribosome. Polyribosomes are attached to the endoplasmic reticulum at its cytosolic face and they eventually form the protein chain. Polypeptide chain requires specific signal sequences that have to be recognized by the endoplasmic reticulum before they can be internalized. The polypeptide sequence is recognised by the presence of signal recognition peptide (SRP). This SRP is recognized by the SRP receptor that is present in the membrane of the endoplasmic reticulum. The SRP binds to the endoplasmic reticulum signal sequence as soon as the peptide has emerged from the ribosome. This binding creates a halt in the protein synthesis and during this pause the ribosome binds to the endoplasmic reticulum. As a result, the polypeptide is not released into the cytosol, thereby preventing proteolytic destruction. The processing of protein is different between transmembrane proteins depending on whether they are single pass or multiple transmembrane proteins. On entering the endoplasmic reticulum lumen the proteins undergo a process of glycosylation to prevent their degradation.
The smooth portions of the endoplasmic reticulum are also involved in protein synthesis, but in a different manner. The smooth areas are those regions in which vesicles are formed that help transport the proteins synthesised in the endoplasmic reticulum to the Golgi complex. In addition to this, the smooth endoplasmic reticulum is important in cells that are actively involved in synthesis of steroids. The smooth region seems to be necessary for incorporation of various enzymes that are required to process cholesterol to form steroids.
In summary, the process of protein synthesis begins in the nucleus, with the conversion of DNA to RNA (transcription). Thereafter, mRNA is converted to the polypeptide chain that is represented by the DNA (translation). The polypeptide chain is transported out through the nuclear membrane pores. It is then processed in the ribosome/ endoplasmic reticulum complex. After this step, it is stored and transported via the Golgi complex into various areas in the cell.
After this brief description of the important organelles of the cell, the rest of the chapter will focus on various cell types of the periodontium.
CELLS OF PERIODONTIUM
The important cells are the keratinocytes, gingival fibroblasts, inflammatory cells, the osteoblasts, osteoclasts, osteocytes of the alveolar bone, periodontal ligament fibroblasts and cementoblasts. Each of these cells serves a unique function and is equipped with features that are required to fulfil these functions. To understand their normal characteristics and the changes they undergo 19is to understand the homeostatic mechanisms of the periodontium and their pathological changes. Many features of these cells have been characterized only recently largely based on their in vitro behavior. It is increasingly accepted that there is a lot of difference in cellular behavior in in-vitro cultures compared to what they really do in the tissue. However, in-vitro results are necessary to understand the behavioral capacity of these cells, at least in experimental conditions.
Keratinocytes
Keratinocytes are the major cell constituents of the gingival epithelium. Keratinocytes are required to provide a sheet of epithelia that act as a barrier to cover underlying vital structures and to fill any breach in the continuity that might occur as a result of physical or a chemical trauma. For these reasons, keratinocytes are closely packed to each other and are capable of rapid motility. These characteristics are conferred to the keratinocytes by the following features.
- Numerous cell-cell contacts that ensure that these cells remain in close contact with each other.
- Contractile elements in the cell cytoskeleton that help in rapid locomotion.
Structurally, keratinocytes are like any other cell in that they have organelles like nucleus, cytosol, ribosomes, Golgi complex etc. The plasma membrane of the keratinocytes is thrown into infoldings on their apical surface. These cytoplasmic extensions aid in the locomotion exhibited by the cells. In gingiva, this movement is essential to replenish the constant shedding of cells that occur in the coronal areas of the gingiva that is exposed to masticatory stress. Intracellular organelles that are present in keratinocytes but not in the other cells of the periodontium, include melanosomes, which are the pigment bearing granules in the keratinocytes. The formation of these granules and their transport into the coronal areas are described in subsequent chapters.
Keratinocytes originate from the ectodermal germ layer. Their differentiation occurs from basal to coronal layers from the epidermal proliferating unit (EPU) present in the basal layers. Keratinization process requires presence of sulfhydryl groups, calcium influx into the cell. Cytokeratins are the characteristic feature of all epithelial cells. The basal cytokeratins are K5 & 14, the simple epithelial keratins are K6, 16, etc. The keratinocytes that are differentiating terminally to produce keratinized epithelium shows K1 & 10. Cytokeratins are also described in detail in later chapters. Other proteins present are the filaggrin, loricin, both of which aid in the process of keratinization.
Gingival keratinocytes, unlike their dermal counterparts, e.g. are also involved in defence mechanisms of gingiva. For defence purposes, keratinocytes of the gingival epithelium secrete antimicrobial peptides. The important among them are defensins. The defensins are classified into α and β defensins. For similar reasons, epithelial cells are associated with inflammatory cells (neutrophils, dendritic cells) and their mediators (IL-8, ICAMs) that modulate immunological reactions that may be required for homeostasis of the gingiva.
Fibroblasts
Fibroblasts are the most important of the mesenchymal cells associated with soft tissues. These cells are a heterogeneous population characterized by their stellate shape and presence of the intermediate filament vimentin on their surface. In the 20periodontium, the fibroblasts of the two soft tissues-gingiva and the periodontal ligament differ from each other so much so that they have to be treated almost like two different cell types. As a result, the fibroblasts of the two tissues are discussed in comparison to each other.
The difference between the two fibroblast populations is related to the functions they are expected to perform. PLF's are primarily responsible for anchoring teeth to bone, absorb mechanical forces and maintain tissue homeostasis during tooth movement. Gingival fibroblasts on the other hand, contribute to immune regulation in addition to production and maintenance of the ECM of gingiva.
Origin
The difference in their origin is largely responsible for the genetic and other structural features of these fibroblasts. In addition, the enviornment in which the two fibroblast population differentiate from their progenitor cells shows considerable difference. The PLF's differentiate in an environment that favors the development of cementoblasts and osteoblasts. Consequently, the signals that they are exposed to differ considerably from that of the GFs that do not receive any signals from putative mineralized tissues. This would also contribute to the phenotypic difference between the PLF's and the GFs.
PLF's originate from stem cells that are present in the paravascular spaces. From these spaces, cells proceed in their differentiation pathway until they become fully functional fibroblasts by moving towards the extracellular spaces. The differentiating cells move in a direction from the central towards the peripheral areas of the PDL space. Similarly, these cells exhibit directional motility in an apicocoronal direction. Gingival fibroblasts, are derived from the undifferen-tiated progenitor mesenchymal cells that are present in the follicle and more importantly from the ridge mucosa.
Morphology
The PLFs and GFs are pretty much similar to each other in gross anatomy. They are spindle shaped cells with a large eccentrically placed nucleus. Cytoplasmic protruberances that resemble pseudopods are present in their cell surface.
The fibroblasts are, however a heterogeneous lot with different sub-populations of cells present in both the gingiva and periodontal ligament. Some of these heterogeneous populations present differences in their morphometry. The PLFs have been shown to exhibit almost 4 to5 different (morphological) shapes ranging from round to stellate shaped. These differences are dictated largely by their differences in functionality and stage of differentiation.
Cell size
In health, the GFs are smaller in size when compared to the PLF's. These differences persist in the wound healing process with the PLF's of the wound space exhibiting a much greater size than the GFs. The increased size of the PLF's means greater intracellular organelle content, especially that which is actively involved in protein synthesis, i.e., greater content of the Golgi/ER/ribosome complex.
Cytoskeletal elements
The PLFs exhibit a much greater expression of αSMA when compared to the GFs. In 21addition, PLF's exhibit the presence of α smooth muscle myosin that is absent in the GFs.
The greater expression of the contractile elements in the PLF's membrane grants these fibroblasts a greater ability to contract collagen. This greater expression of contractile proteins would have functional relevance to the PDL as they are better equipped to absorb mechanical stress without being displaced from the socket. The stretching and elongation of the collagen fibers can be thus offset by the contraction of the PLF's.
Similarly, the cytoskeleton related proteins such as Transglein and Desmoplakin are expressed to a greater extent in the PLFs. Transglein is a surface protein that is thought to be associated with and upregulated in cells undergoing shape transformation. They are found usually associated with the actin filaments. Along with the contractile elements, these proteins help the PLFs change shape at will. This is an important functional adaptation that enables PLFs to accommodate the stretching and compression of the collagen bundles along which these cells are lined.
Cell proliferation and apoptosis
The GFs exhibit a greater proliferative rate than the PLFs. Even among the GFs, there is some heterogeneity in their proliferation rate. The smaller sized GFs are known to exhibit an even greater rate of proliferation, especially in those tissues with overgrowth.
A greater expression of the cell cycle associated proteins such as Cyclins observed in the GFs are thought to aid in this increased proliferation. This greater proliferation has an important clinical relevance in that these cells are able to provide enhanced wound fill. Greater proliferation is linked to several genetic and environmental factors as distinct subsets within the gingiva exhibit greater capacity to proliferate.
On the other hand, apoptosis occurs at a much lower rate in the PLF's when compared to the GFs. The presence of the SFRP (Secreated Frizzled Related Protein) that activates the WNT pathway may act along the GBP(i) especially to retard apoptosis. The longer cell cycle of the PLFs means that these cells have the ability for greater protein synthesis and thus matrix turnover:
Functions and structural proteins
The GFs function primarily for the production of the ECM and synthesizes collagen in response to growth factors such as the CTGF, TGF β etc. They are also important accessory immune cells in the periodontal environment. They, therefore, exhibit a greater expression of CD40 which is used to bind to the Tcells that have the CD40L. This CD40 mediated binding has important implications in the regulation of immune responses. Greater expression of S100A and Periostin in the PLF's has been observed and these proteins can be used as markers for PLFs.
Cultured gingival and periodontal fibroblasts differ in their protein synthesis. Periodontal fibroblasts are thought to exhibit greater quantities of protein related to extracellular matrix, especially collagen I, sulphated glycosaminoglycans, fibronectin, vitronectin and laminin when compared to the gingival fibroblasts. Gingival fibroblasts are capable of greater expression of angiogenic factors Ang-1, VEGF, when compared to periodontal fibroblasts. Periodontal fibroblasts show greater expression of the negative regulator of angiogenesis namely the pigmented epithelial derived growth factor (PEDF). Among the various non-collagenous proteins secreted by the fibroblast, gingival 22fibroblasts show the greater expression of the elastin protein, secreting elastin, elaunim and oxytalan. The periodontal fibroblasts are capable of secreting only the oxytalan fibers.
Trans membrane proteins
The transmembrane proteins that are involved in the transport of sulfates and proteins in and out of the cell, such as the SLC gap of proteins are upregulated in PLFs when compared to GFs. So also with the adhesion proteins such as MFG E8 which are involved with the substrate adhesion. Additionally, the integrin expression in these fibroblasts is different from each other and even more so when compared to other fibroblasts such as dermal fibroblasts. Both fibroblasts exhibit the collagen binding Integrins such as α2β1 and α1β1, but they are in greater concentration in the GFs. The PLFs exhibit greater expression of the Laminin binding integrin α3β1. The Fibronectin binding α5β1 are present in both fibroblasts.
Both fibroblasts are involved in catabolism of connective tissue elements such as collagen. Fibroblasts are involved in phagocytosis of collagen by two different mechanisms.
- They internalize collagen through integrins such as α2β1 and α1β1. The collagen then undergoes digestion intracellularly through various enzymes such as cathep-sins present in the lysosome of the cell.
- In addition to this, fibroblasts are also involved in collagen degradation through secretion of proteolytic enzymes, the matrix metalloproteinases (MMP). The collagenase MMP-1, the fibroblast type collagenase is secreted by the fibroblasts. These MMPs are involved in breakdown of type I collagen into a ¾ and ¼ segment. Further breakdown of collagen occurs through the action of other MMPs such as gelatinases, matrilysins, etc.
The periodontal fibroblasts are also capable of exhibiting greater alkaline phosphatase (ALP) activity than the gingival fibroblasts. Alkaline phosphatase is an essential requisite for formation of mineralized tissue as it is necessary for conversion of organic phosphates to inorganic phosphates prior to formation of hydroxyapatite
Periodontal ligament fibroblasts
Not all periodontal ligament fibroblasts are similar in nature. They are a heterogenous lot that differ in motility, collagen I synthesis and fibronexus formation. Periodontal ligament fibroblasts are basically of two lineages-fibroblastic lineage and osteoblastic lineage of cells. The fibroblastic lineage of cells are involved in collagen synthesis and do not reveal any capacity to form mineralized tissue. These cells maintain ‘steady stream’ in that they are relatively slow to divide. These steady stream of cells that belong to the fibroblastic lineage do not under normal circumstances form any kind of mineralized tissue. These cells contribute to maintenance of the periodontal ligament space. The other cell lineage is the osteoblastic lineage of cells. These cells are more rapidly proliferative in nature and under specific culture conditions are capable of transformation to osteoblasts if they are exposed to dexamethasone, β-glycero-phosphate and ascorbic acid. Surface receptors are also differentially expressed by the heterogenous periodontal ligament fibroblasts. The cells of the osteoblast lineage have a higher expression of fibroblast growth factor receptor FGFR2. This receptor induces osteoblastic differentiation of the osteoblastic lineage of the heterogenous populations of periodontal ligament fibroblasts.23
Periodontal ligament fibroblasts can also transform to bone forming cells when they are exposed to constant mechanical stress. The periodontal ligament fibroblasts exhibit special features so as to facilitate their osteoblastic behavior. The periodontal ligament fibroblasts, exhibit a baseline expression of Cbfa1, even under unstimulated conditions. Cbfa1 is a master switch that regulates osteoblastic differentiation. The baseline Cbfa1 expression of the periodontal fibroblasts is definitive indication of the ability of these cells to undergo osteoblast differentiation. In addition, these cells can also exhibit a higher level of bone morphogenic proteins (BMPs)-2 and 4, both of which are required for bone formation, than the gingival fibroblasts. These cells are also capable of higher expression of bone related proteins such as osteopontin (OPN) and osteonectin (ON). However, Bonesialoprotein (BSP) the initial nucleator of hydroxyapatite crystals is minimally expressed by the periodontal ligament fibroblast. Osteocalcin (OCN), the regulator of crystal formation is however highly expressed in periodontal ligament.
Cementoblasts
Cementoblasts are the least understood of all the periodontal cells. The difficulty of culturing cementoblasts from the mineralized cementum has prevented their complete characterization. Cementoblasts from the other sources have been immortalized from knock out mice and are designated as OCCM. These cells have been used to understand the gene profile and biological nature of cementoblasts.
Origin
The origin of the cementoblasts that form the acellular cementum and cellular cementum are thought to be different from each other.
Acellular cementum
Cementoblasts are known to differentiate from the dental follicle cells of the dental sac. After crown formation is almost complete, the outer and inner enamel epithelium fuses to form the reduced enamel epithelium (REE). This REE forms the cervical loop, which then differentiates to form hertwigs epithelial root sheath (HERS). Developing root is formed from the HERS. Traditionally, the undifferentiated cells of the dental follicle were thought to transform into cementoblasts.
The epithelial mesenchymal inter-relationship that goes towards formation of enamel/dentine with the inductive role played by odontoblasts in formation of enamel, is clearly understood. However, it was realized in recent years that epithelium may play a role in formation of cementoblasts as well. The cells of the HERS secrete proteins that have been collectively termed as the enamel matrix proteins, the principal proteins amongst which are amelogenin, sheathlin and tubulin. These enamel proteins play an inductive role in differentiation of developing undifferentiated mesenchymal cells to form cementoblasts. These enamel derivatives form the matrix and secrete inductive factors that cause undifferentiated mesenchymal cells to form cementoblasts.
In recent years, another hypothesis regarding the origin of cementoblasts has been proposed. Cell culture studies have revealed that there is a propensity for some cell types to undergo transformation into a cell of a different germ line, e.g. Epithelial cells cultured in collagen gels have been shown to transform to cells that resemble myofibroblasts. Other cells have also shown a similar propensity and this process is thought to be important in the wound healing process. This phenomenon has been termed 24epithelial-mesenchymal transformation or trans-differentiation.
Bosshardt and Nanci have proposed that the cells of Hertwigs epithelial root sheath may show a similar effect and transform to form cementoblasts. Earlier investigators reported that these cells have shown secretory vesicles on their surface that face the forming cementum that were thought to secrete the amelogenin related proteins. These authors suggest that these cells show a change in morphology as cementum formation proceeds. This has led them to propose that it is actually the HERS cells that trans-differentiate to form cementoblasts. Further strength to this theory has come from studies that have reported that REE (the resting cells of the HERS) seems to be involved in cementum repair. These cells have also shown evidence of presence of bone related proteins such as osteopontin, bonesialoprotein and the related BMPs such as BMP-2.
Cellular cementum
Cellular cementum on the other hand is derived from cells that arise probably from the paravascular areas of the PDL. These cells are now known to be distinct from the osteoblasts.
Morphology
The cementoblasts that form the cellular cementum and acellular cementum differ from each other in shape. The cementoblasts that form C1FC (the type of Cementum that resembles bone most) are large cuboidal cells with a single bound nucleus. The usual organelles associated with protein synthesis and transport-the ribosome/ ER/Golgi complex are well developed in these cells. These cells, like osteoblasts are entrapped in a mineralized cementoid matrix as a result of rapid deposition of the mineralized matrix. Not only is the matrix rapidly laid down, it occurs in a multinodal manner, i.e., there are several centers of nucleation. Consequently, the cells are enclosed within a matrix that is laid down in different centres within the cementum.
The morphology of the cementoblasts that lay down AEFC is similar to the PLFs, so much so that they are virtually indistin-guishable from each other. As suggested earlier, heterogenecity of the PLFs means that there are several cell types that are structurally distinct from each other. Cementoblasts resemble only those PLFs that line the cementum surface and seem to be regulating cementogenesis.
Under the SEM, the cementoblasts has been shown to have cytoplasmic extensions that have been differentiated into wing like processes and finger like processes. The wing like processes encircle the extrinsic fibers while the finger like processes line the cementum surface and are involved in intrinsic fiber production. The intrinsic fibers are parallel to the root surface and are thought to form a link between the extrinsic fibers and the root surface.
Regulation
Several systemic and local factors contribute to differentiation of cementoblasts and to the regulation of their functions.
Systemic
PTH/Pthrp – Cementoblasts exhibit receptors for these proteins and they regulate the production of both CIFC and AEFC.
GH – Cementoblasts of the CIFC are positively influenced by this harmone
Local regulators
OPG is increased in the periodontal ligament preventing cementum resorption
Cytokines such as PDGFBB, FGF, and TGFβ and IL-1 all regulate CIFC, while PGE 2 shows a biphasic response.
Amelogenins are one of the principal local regulators of AEFC.
Amelogenin
The inductive role of EMPs in the differentiation of cementoblasts is now well established. The most abundant among the EMPs is amelogenin that constitutes 90% of the total protein content. It has been demonstrated that these Amelogenins may have a role in the differentiation of Cementoblasts forming Acellular cementum. Amelogenin is however not unidimensional in its effect on mesenchymal cells. There is considerable difference in its effect as a whole protein and as splice variants of the original structure.
Amelogenin gene consists of 7 exons and introns in between this and an active ‘amino’ terminal end that is designated as the TRAP (tartarate rich amelogenin protein). TRAP has the ability to upregulate pathways that are involved in Cementoblast differentiation, for eg., Cbfa1, BSP, etc. Another member of this family LRAP is also known to upregulate the Cementoblast differentiation process.
Other splice variants of the Amelogenin gene designated A+4 and A-4 are known to differentially regulate the mineralization process. A-4 is specifically thought to upregulate Cbfa1 expression, therefore helping the mineralization process while A+4 causes collagen II expression regulating osteoblast differentiation.
Protein secretion
Cementoblasts are capable of secreting most of the proteins associated with mineralized tissue such as bonesialoprotein, osteocalcin and osteopontin. However, cementoblasts inherently do not exhibit alkaline phosphatase activity. Alkaline phosphatase required for the formation of cementum is instead obtained from the periodontal ligament cells that are in close association with the developing cementum. Cementoblasts are also associated with, as in bone forming cells, a high expression of Cbfa1, collagen I genes.
Cementoblasts, like the osteoblasts, show surface integrins that are required for attachment to collagen. Mineralization is a process that occurs after cells are adherent to collagen I. The surface integrins are α2β1 and those that are attaching to the bone associated proteins. Numerous β3 integrins are present on the surface of cementoblasts. The αvβ3 integrins help them attach to osteopontin, bonesialoprotein and osteonectin.
There are several proteins in the matrix that are unique to cementum. The keratan sulphate rich lumican is present in greater concentration in cementum than in bone. This proteoglycan is secreted by the cementoblasts. However, the process involved in the regulation of their secretion is yet to be fully understood. Additionally, cementoblasts secrete and respond to a unique protein called the cementum attachment protein (CAP). This CAP is thought to play a role in recruitment of cementoblasts to the developing cementum. Further, CAP is important for insertion and retention of periodontal ligament fibers. CAP is secreted by the cementoblasts and again the genes regulating this process and the growth factors responding to their activation is not yet fully understood.
In any case, there is still some work to be done before the complex mechanisms that underlie the origin of cementoblasts, the protein they secrete and their ultimate 26organization into the mineralized tissue can be fully unravelled. It is therefore, no surprise that of all the tissues of periodontium, cementum continues to be the least regenerated.
Bone forming cells
The cells of the bone are the osteoblasts, osteoclasts and osteocytes. Osteoblast is the bone forming cell that helps in the formation of the mineralized tissue in bone. Osteoclast, on the other hand, is the bone resorbing cell, that causes demineralization. Osteocyte is the resting cell whose role in homeostasis of the bone has been increasingly recognized in recent years.
Bone is a mineralized tissue that is in a constant state of flux. Since it is bone that is responsible for all the mechanical forces applied, it is important that it is a dynamic structure that is capable of constant change. The alveolar bone is always exposed to masticatory forces, necessitating constant formation and resorption. This is made possible only through the presence of osteoblast/osteoclast coupling reaction. In other words, these two cells are dependant on each other for their existence and for efficient functioning. Alveolar bone, in addition, also has to contend with a constant inflammatory reaction that originates from gingiva. The inflammatory reaction of the alveolar bone has to be tightly regulated to prevent excessive loss of bone. Both osteoblasts and osteoclasts have to act in tandem to form and maintain homeostasis of bone.
Osteoblast
Osteoblasts are the major bone forming cells that help form mineralized tissue. Osteoblasts originate from stromal cells that are present in the bone marrow. These cells are however multipotent in nature and can differentiate into various cell types such as the myocyte, adipocte, chondrocyte or the osteoblast. The cell type they can differentiate into depends on the transcription factors that are available to the BMSC's, e.g. on exposure to MyOD they differentiate to form the myoblast, whereas when exposed to PPARγ, these cells transform to form adipocytes.
The definitive transformation of BMSC's into future osteoblasts occurs when they are in the presence of Runx2/Cbfa1. The runt homology regions have the requisite genetic material that can result in the activation of its own gene. On activation of the Cbfa1 gene, other genes that are associated with bone formation can further be activated. Important among these are the ColI gene, the ALP gene and genes for specific bone related proteins such as OPN, BSP and importantly OCN. There are other genes that are required for osteoblast activation in their early stage. Important ones are the TAZ gene; Dlx homeobox genes that help transform the early uncommitted progenitor cells to become committed osteoblast progenitors. Once the cells become committed osteoblasts, other factors such as the Cbfa1 and osterix are responsible for the differentiation of these cells to form the fully developed osteoblast.
Structure
Osteoblasts are large cuboidal cells that form a single layer on endosteal or periosteal surfaces. They have a round nucleus and all the organelles required for protein synthesis – Golgi/ER/ribosome complex.
Lining cells are osteoblasts that have a flat shape and limited intercellular organelles within it.27
Osteoclast
Osteoclasts originate from the monocyte-macrophage line of cells. The monocyte-macrophage lines of cells gradually coalesce and form the multinucleated osteoclast. Osteoclasts are present in specific areas in the bone called the Howship's lacunae. These cells exhibit a ruffled border because their cytoplasmic membrane is thrown into several folds.
Origin
Osteoclasts are derived from the Hematopoitic Stem Cell (HSC) which transform into monocyte macrophage cells and then form osteoclasts. MCSF causes the HSC to be pushed into the myloid precursor line. Once this precursor line is established, another factor called PU − 1 aids this process. The c-fos of the AP1 is also an important determinant of osteoclast activation. Additionally, the MAPK pathway is activated where the JNK and p38 are chiefly involved. RANK – RANKL signaling is then important for causing the myeloid cell to transform to the osteoclast cell lineage. Ultimately, activation of NFATc1 acts as the master switch that regulates osteoclast differentiation.
Once these signals are in place, fusion of multiple cells must occur for the formation of the multinucleated osteoclasts. Fusion consists of two processes:
- Focal lysis of the membranes on cells, so that they can fuse at these spots. This lytic process is performed usually at the instance of the disintegrins and ADAMs that are capable of proteolysis of the cell membrane.
- Molecules that are actively involved in two or more cells in adhering to each other. One such identified protein is Syncitin, and CD4, other macrophage protein such as SHIP may also be involved along with the SHPS − 1 receptor.
Osteoblast-osteoclast coupling
As was previously indicated, the osteoblast-osteoclast development is interdependent on each other. The development of osteoclasts is controlled by the stromal cells through the RANK/ RANKL/OPG axis. The developing osteoclasts have the RANK receptor on its cell surface. Activation of this receptor is essential for the activation of osteoclasts through the NFATc-1.
RANK is activated through its ligand RANKL. RANKL is produced from the osteoblasts/stromal cells. Upon RANK/RANKL binding, activation of osteoclasts occurs and subsequently leads to bone resorption. On the other hand, osteoprotegrin (OPG) is also produced by various mesenchymal cells and acts as a soluble decoy receptor for RANKL. OPG binds to RANKL but prevents downstream activation. As down stream signaling does not occur, there is no activation of transcription factors and therefore no osteoclastogenesis. Apart from the osteoblasts, other source of RANKL is the activated T cell. The link between the immunological reactions and osteoclasto-genesis has been identified in recent years. This understanding led to the development of a new branch “Osteoimmunology”. This finds relevance in several diseases such as rheumatoid arthritis, where the activation of T cells in synovial spaces is thought to lead to excessive RANKL production and osteoclast activation, leading to bone loss observed in this disease.
The RANK/RANKL/OPG axis has special significance in the periodontium. The periodontal ligament fibroblasts have been demonstrated to produce high levels of OPG 28in health. This expression of OPG prevents excessive osteoclastogenesis and thereby prevents root resorption. In periodontal disease, it has been observed that T cells present in the gingival tissues can produce RANKL. It is thus thought possible that osteoclastogenesis observed in periodontal disease is a direct result of RANKL production by the T cell. Either way, it is clear that osteoclast development and activation is atleast in part dependant on signals produced by the osteoblasts. Conversely, the osteoblast activation is also dependant on osteoclasts. The fully developed osteoclast has abundant lytic enzymes like the TRAP. This acid phosphatase results in demineralization of the mineralized tissue. This results not only in bone loss, but also release of inductive factors present in the matrix including the BMPs. BMPs are important for osteoblast development and are entrapped in the bone matrix. Liberation of BMPs from the mineralized matrix, results in further osteoblast differentiation and this activates Cbfa1 and other genetic factors responsible for osteoblast development and maturation.
Bone, in addition to providing mechanical rigidity to enable locomotion, is also an important store house of calcium. Calcium signaling is important as a second messenger for a number of cellular reactions that are important to sustain life. When serum calcium levels undergo any change, the calcium required for replenishing the reduced calcium is obtained from bone. On the contrary, when excess calcium is present, it is excreted by the kidney with sufficient reservoirs stored in bone.
Parathormone is the major hormone that regulates serum calcium levels. The receptor for parathormone is present on osteoblasts and not the osteoclasts. In response to the parathormone stimulus, osteoblasts release greater quantities of RANKL resulting in osteoclastogenesis. The mandible may also serve as a reservoir even though it may not be the primary calcium storing organ like the long bones. Thus, in conditions like hyperparathyroidism, the mandible may also lose mineralized tissue, resulting in the condition called Paget's disease of the bone (osteitis fibrosa cystica).
Locally generated inflammatory mediators such as IL-6 do not affect the osteoclasts directly. The IL-6 receptor is present on the osteoblasts, which on activation results in increased production of RANKL which subsequently results in osteoclastogenesis. In summary, both systemic and locally generated signals that result in formation and destruction of bone do so through stimulation of the osteoblast/osteoclast coupling behavior.
Osteocytes
Osteocytes contribute to about 90–95% of the cells of the bone. In spite of being the most abundant among the bone cells, it is only recently that the role played by these cells is being clarified. These cells were previously thought to be merely ‘resting cells’, i.e., osteoblasts that are in a post functional state presumably not involved in the homeostasis of bone. It is only recently that its role in maintaining bone homeostasis and mechanosensation is being recognized.
Osteocytes present in the mineralized matrix embedded in ‘cases’ that are called lacuna. These cells have long cytoplasmic processes called canaliculi through which they communicate with each other and the other bone cells. Through these communications, they are in a position to direct osteoblast/osteocyte activity on 29demand so as to regulate mineralization. Osteocytes are generally identified by the presence of MEPE, Sclerostin/E11/qp28 all of which are specific to these cells.
Communication in osteocytes
The major form of cell communication in osteocytes occurs as follows:
- Gap proteins mediated through the connexons.
- Fluid transfer within the canaliculi
These communications are thought to allow the osteocytes to transmit signals they receive from the external environment to the other cells of bone, thereby making them respond in order to maintain homeostasis of the bone.
For a long time, it was thought that osteocytes are quiescent cells that do not play any specific role within bone. It is now realized that osteocytes have three important functions:
- Regulate the bone mass and thus the shape of the bone.
- Mechanosensing within the bone
- Help regulate osteobast/osteocyte activity.
Osteocytes have surface receptors such as Integrins (αvβ3); gap junction proteins connexins and canaliculi that respond to fluid movement that corresponds to mechanical stress.
On loading, both anabolic and catabolic responses occur.
Anabolic responses
There is an upregulation of intracellular pathways such as those associated with c-fos and IGF mediated pathways. This results in upregulation of proteins such as DMP 1 and MEPE – both of which can increase osteoblast differentiation. The membrane associated E11/gp38 is increased, resulting in formation of canaliculi in the osteocytes. This dendritic elongation causes a greater ability for osteoblasts to respond to osteocyte stimuli. The other important gene that is activated following mechanical loading is Sclerostin that can be a negative regulation of mineralization.
Catabolic responses
Increased RANKL activity has been demonstrated following loading as a result of which there is osteoclast activation.
The two primary genes that respond to mechanical loading are
The Wnt catenin pathway that increases mineralization.and
LRP 5 which is an important positive regulator of mechanically stimulated gene activity. Sclerostin, on the other hand is the most important negative regulator that responds to membrane loading.
Osteocyte is hence, far from a passive cell, it plays an important role in mechano-sensation and regulation of mineralization.
In addition to these important cells that go to form gingiva, periodontal ligament, cementum and alveolar bone, there are a number of other cells that play a role in maintaining the homeostasis of the periodontium. Important among these cells are the inflammatory cells, both of innate and adaptive immune systems. Inflammatory cells and their actions are beyond the scope of this book. Other cells, unique to the periodontium are the epithelial cell rests of malassez, melanocytes and the undifferentiated mesenchymal cells.
Epithelial cell rests of malassez
Epithelial cell rests of malasisez (ECRM) are the only epithelial cells of the periodontal ligament. These cells are the remnants of 30hertwig's epithelial root sheath (HERS) that are left behind after root formation is complete. Epithelial cell rests of mallasez are present as strands or cords of cells that are present in the periodontal ligament, usually present in the coronal aspects of the root rather than in the apical areas. It has been suggested that they form a sort of a basket in the periodontal ligament arising from the cementoenamel junction and are suspended from there in a loop like manner.
Several theories have been forwarded regarding their function or the lack of it. It has been proposed that epithelial cell rests of malassez play an important role in the maintenance of the periodontal ligament space. In physiological conditions, it is therefore thought that these cells that are devoid of any mineralization propensity may contribute to maintaining a non-mineralized area. Some authors have shown that these cells may lead to ankylosis of teeth in isolated areas.
Structurally, epithelial cell rests of malassez are similar to epithelial cells anywhere else. The cytokeratin profile of these cells is similar to that of junctional epithelium, with a high expression of K5, K6, K7, K8, K16 and K19, all of which are characteristic of simple and differentiated epithelium. However, these cells are of ectomesenchymal origin and hence retain some characteristics of odontogenecity. Therefore, it has been reported that these cells show expression of BMP2, 4 and also some bone related proteins such as osteopontin and bonesialoprotein. It has consequently been argued that these cells may be involved in regeneration of periodontium, especially cementum. These cells are involved in pathogenesis of several disorders including pocket formation. The presence of c-met receptors in the epithelial cell rests of malassez suggest that these cells can respond to the inflammatory cytokine, Hepatocyte growth factor. This scatter factor is thought to be capable of aiding migration of these cells and hence aid in the pathogenesis of pocket. Also, these cells have been reported to be involved in production of neuropeptides such as substance P, VIP and CDGRP. These neuropeptides are involved in vasodilation and other proinflammatory responses.
These cells are also thought to be responsible for cementum repair as they are found in areas of resorption when mechanical injury was created in experimental animals. In this experimental report, these cells were found to exhibit the presence of BMP2 – an osteoblast inducing factor. It has been suggested that cells retain the capacity to differentiate into cementoblast and lay down matrix.
In summary, the epithelial cell rests of malassez are thought to play a role in physiological maintenance of periodontal ligament space; may play a role in pathogenesis of pocket formation and probably may mediate process involved in regeneration.
Understanding the structure, biology and functions of each of these cell types is an essential step to our understanding of the physiology of the periodontal tissue. The role each cell plays in maintaining the harmony of periodontal tissue needs to be clearly delineated. It is only then that complete regeneration, that remain an elusive goal till now, may become a reality.
Stem cells
Stem cells are undifferentiated cells that have the potential to differentiate into several adult cell types. Stem cells are broadly classified into embryonic stem cell and somatic stem cell. Embryonic stem cells are totipotent in nature, 31i.e., they are capable of transformation into every cell in the body. Adult stem cells, on the other hand, are not capable of complete totipotency. They are multipotent, i.e., can transform into different cells in the body, but not every cell type.
Stem cells are characterised by their ability to divide continuously, in theory, an unlimited number of times. In practice, stem cells do have a finite number of divisions. They are also characterised by a slow turnover rate. The ability to divide continuously when compared to adult stem cells is conferred by the higher expression of teleromase enzyme. The best characterized stem cells are the bone marrow stem cells (BMSC) and the MSCs. BMSC's are identified by the presence of STRO-1 and CD172. BMSC's differentiate into adipocytes, monocytes, osteoblasts or blood cells based on the availability of the transcription factors.
The presence of stem cells in periodontal ligament has been under investigation for a very long time. Progenitor cells in the paravascular spaces of the periodontal ligament have demonstrated the capacity to differentiate into osteoblasts and periodontal ligament fibroblasts. Stem cells have been recently identified in the periodontal ligament with the marker STRO-1. These cells have shown to differentiate into adipocytes, fibroblasts and osteoblast and form mineralized nodules. It is this ability of the stem cells to differentiate into adult cell types that make these cells an attractive option for use in tissue engineering techniques and regenerative procedures.
The lack of appropriate delivery systems, i.e., vehicles through which stem cells can be implanted to the desired site limit their clinical use to date. The development of better biological systems that provide the right matrix/ growth factors / adhesion molecules that can deliver these stem cells into vertical defects can result in optimal regeneration. Although much work remains to be done, it is clear that identification of stem cells have paved the way for development of newer techniques in periodontal regeneration.
SUMMARY
Keratinocytes, fibroblasts of the gingiva and periodontal ligament, cementoblasts and the bone forming cells are the basic units that form the tissues of periodontium. A thorough understanding of the molecular events in these cells would provide a deeper insight into the processes that maintain the homeostasis of the periodontium. Pathological changes that affect the periodontium are a result of not only the invading microrganisms, but also a behavioral change in the cellular metabolism.
A thorough understanding of these cellular changes would also help evolve strategies that could revert these pathological changes. Knowledge of cellular behavior is also important to devise newer treatment strategies that involve tissue engineering techniques. Stem cell research is currently thought to be the most promising method for regenerating lost tissue parts.
BIBLIOGRAPHY
- Ainamo A. Influence of age on the location of the maxillary mucogingival junction. J Perio Res 1978; 13: 189–93.
- Alatli, Lundmark C, Hammarström L. The localization of epithelial root sheath cells during cementum formation in rat molars. J Perio Res 1996; 31: 433–40.
- Alberts, Johnson, Lewis, Roff, Roberts, Walter. Molecular biology of the cell 4th edition 2002.
- Araujo RMS, Oba Y, Moriyama K. Identification of genes related to mechanical stress in human periodontal ligament cells using microarray analysis. J Perio Res 2007; 42: 15–22.
- Bartold M, Lawrence WJ & Narayanan AS Molecular and cell biology of the gingiva. Periodontol 2000 2000; 24: 28–55.
- Bartold PM and Narayanan AS Biology of Periodontal Connective Tissue, 1998. Quintessence publication Inc.
- Bosshardt. Are Cementoblasts a Subpopulation of Osteoblasts or a Unique Phenotype? J Dent Res 2005; 84: 390–406.
- Bostancý N, Ýlgenli T, Emingil G, Afacan B, Han B, Töz H, Berdeli A, Atilla G, McKay IJ, Hughes FJ, Belibasakis GN. Differential expression of receptor activator of nuclear factor-êB ligand and osteoprotegerin mRNA in periodontal diseases. J Perio Res 2007;42:287–93.
- Chen S, Marino V, Gronthos S, Bartold PM Location of putative stem cells in human periodontal ligament. J Perio Res 2006;41:547–53.
- Dale BA. Periodontal epithelium: A newly recognized role in health and disease. Periodontol 2000 2002;30:70–8.
- Dieter D. Bosshardt and Antonio Nanci. Immunolocalization of Epithelial and Mesenchymal Matrix Constituents in Association with Inner Enamel Epithelial Cells. J Histochem Cytochem 1998;46:135–42.
- Dummett O. Physiologic pigmentation of the oral and cutaneous tissues in the negro. J Dent Res 1946;25:421–32.
- Fujita T, Iwata T, Shiba H, Igarashi A, Hirata R, Takeda K, Mizuno N, Tsuji K, Kawaguchi H, Kato Y, Kurihara H. Identification of marker genes distinguishing human periodontal ligament cells from human mesenchymal stem cells and human gingival fibroblasts. J Perio Res 2007; 42:283–86.
- Garant P. R. Oral Cells and Tissues, 2003, Quintessence publishing Co, Inc.
- Gronthos S, Akintoye O, Wang C & Shi S. Bone marrow stromal stem cells for tissue engineering. Periodontol 2000 2000; 41: 188–95.
- Hasegawa N, Kawaguchi H, Ogawa T, Uchida T, Kurihara H. Immunohistochemical characte-ristics of epithelial cell rests of Malassez during cementum repair. J Perio Res 2003;38:51–6.
- Hatakeyama J, Philp D, Hatakeyama Y, Haruyama N, Shum L, Aragon MA, Yuan Z, Gibson CW, Sreenath T, Kleinman HK, and Kulkarni AB. Amelogenin-mediated Regulation of Osteoclastogenesis, and Periodontal Cell Proliferation and Migration. J Dent Res 2006;85:144–49.
- Hughes J, Turner W, Belibasakis G & Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol 2000 2000; 41: 48–72.
- Ivanovski S, Komaki M, Bartold M, Narayanan AS. Periodontal-derived cells attach to cementum attachment protein via α5β1 integrin. J Perio Res 1999;34:154–159.
- Julio C. Rincon, Yin Xiao, William G. Young, P. Mark Bartold. Production of osteopontin by cultured porcine epithelial cell rests of Malassez. J Perio Res 2005; 40: 417–26.
- M. Kagayama, HC Li, J Zhu, Y Sasano, Y Hatakeyama, I. Mizoguchi. Expression of osteocalcin in cementoblasts forming acellular cementum. J Perio Res 1997; 32: 273–78.
- Masaru Kaku, Katsumi Uoshima, Yasuo Yamashita, Hiroyuki Miura. Investigation of periodontal ligament reaction upon excessive occlusal load – osteopontin induction among periodontal ligament cells. J Perio Res 2005;40: 59–66.
- Mc Culloh, Lekic P & McKee D. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol 2000 2000; 24: 56–72.
- Myokai F, Oyama M, Nishimura F, Ohira T, Yamamoto T, Arai H, Takashiba S, Murayama Y. Unique genes induced by mechanical stress in periodontal ligament cells. J Perio Res 2003; 38: 255–61.
- Nakajima R, Yamaguchi M, Kojima T, Takano M, Kasai K. Effects of compression force on fibroblast growth factor-2 and receptor activator of nuclear factor kappa B ligand production by periodontal ligament cells in vitro. Journal of Periodontal Research 2008; 43: 168–73.
- Newman, Takei, Klokevold, Carranza. Clinical Periodontology Tenth Edition 2006. Saunders Publictions.
- Ohshima M, Noguchi Y, Ito M, Maeno M, Otsuka K. Hepatocyte growth factor secreted by periodontal ligament and gingival fibroblasts is a major chemoattractant for gingival epithelial cells. J Perio Res 2001; 36: 377–83.
- Ohshima M, Tokunaga K, Sato S, Maeno M, Otsuka K. Laminin-and fibronectin-like molecules produced by periodontal ligament fibroblasts under serum-free culture are potent chemoattractants for gingival epithelial cells J Perio Res 2003; 38: 175–81.
- Ozaki S, Kaneko S, Podyma-Inoue KA, Yanagishita M, Soma K Modulation of extracellular matrix synthesis and alkaline phosphatase activity of periodontal ligament cells by mechanical stress. J Perio Res 2005; 40: 110–17.
- Ozaki S, Kaneko S, Podyma-Inoue KA, Yanagishita M, Soma K. Modulation of extracellular matrix synthesis and alkaline phosphatase activity of periodontal ligament cells by mechanical stress. J Perio Res 2005;40: 110–7.
- Pi S, Lee S, Hwang Y, Choi M, Lee S, Kim E. Differential expression of periodontal ligament-specific markers and osteogenic differentiation in human papilloma virus 16-immortalized human gingival fibroblasts and periodontal ligament cells. J Perio Res 2007; 42: 104–13.
- Pitaru S., Narayanan SA, S Olson, Savion N, Hekmati H, Alt I, Metzger V. Specific cementum attachment protein enhances selectively the attachment and migration of periodontal cells to root surfaces. J Perio Res 1995;30:360–68.
- Ricardo D Coletta, Egdard Graner. Hereditary Gingival Fibromatosis: A Systematic Review. J Periodontol 2006;77:753–764.
- Saygin NE, Giannobile W, & Somerman J Molecular and cell biology of cementum. Periodontol 2000 2000; 24:73–98.
- Schroeder E and Theilade J. Electron microscopy of normal human gingival epithelium. J Perio Res 1966;1:95–119.
- Schroeder E. Melanin containing organelles in cells of the human gingiva I. Epithelial Melanocytes. J Perio Res 1969;4:1–18.
- Sodek J & Mckee D. Molecular and cellular biology of alveolar bone. Periodontol 2000 2000; 24: 99–126.
- Suzuki M, Matsuzaka K, Yamada S, Shimono M, Abiko Y, Inoue T. Morphology of Malassez's epithelial rest-like cells in the cementum: transmission electron microscopy, immuno-histochemical, and TdT-mediated dUTP-biotin nick end labeling studies J Perio Res 2006;41:280–7.
- Tahiro K, Sawada T, Inoue S, Yanagisawa T Development of oxytalan fibers in the rat molar periodontal ligament. J Perio Res 2002,37:345–52.
- Takayama S, Murakami S, Nozaki T, Ikezawa K, Miki Y, Asano T. Terashima A, Okada H. Expression of receptors for basic fibroblast growth factor on human periodontal ligament cells. J Perio Res 1998;33:315–22.
- Takayanagi H. Inflammatory bone destruction and osteoimmunology. J Perio Res 2005;40:287–93.
- Ten Cate. Oral Histology. Development, Structure and Function. Antonio Nanci 6th edition, 2003.
- Wada N, Maeda H, Tanabe K, Tsuda E, Yano K, Nakamuta K, Akamine A. Periodontal ligament cells secrete the factor that inhibits osteoclastic differentiation and function: the factor is osteoprotegerin/osteoclastogenesis inhibitory factor. J Perio Res 2001; 36: 56–63.
- Wilson N, Schmid MJ, Marx DB, Reinhardt RA Bone turnover markers in serum and periodontal microenvironments. J Perio Res 2003;38:355–61.
- Yoshinaga Y, Ukai T, Abe Y, Hara Y. Expression of receptor activator of nuclear factor kappa B ligand relates to inflammatory bone resorption, with or without occlusal trauma, in rats. J Perio Res 2007;42:402–9.
- Yoshino H, Morita I, Murota S, Ishikawa I. Mechanical stress induces production of angiogenic regulators in cultured human gingival and periodontal ligament fibroblasts. J Perio Res 2003;38:405–10.